With its systematic layout, the periodic table makes it easier to learn about the many chemical elements.
Chemists benefit from knowing the rationale for these elemental reactions. Each of the more than a hundred elements has a unique symbol in the periodic table.
To further understand the arrangement of the elements, we’ve included a small sample of the periodic table below. The periodic table of elements is organised from atomic number one to atomic number 92.
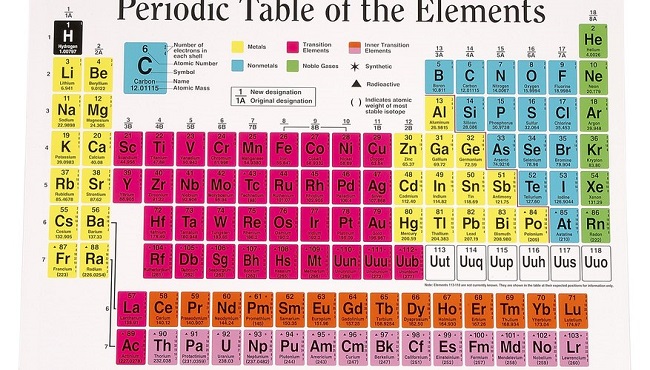
Periods are used to describe the left-to-right rows, whereas groups are used to describe the right-to-left columns. The chemical characteristics of elements within the same group are highly consistent.
This is due to the fact that they share the same valency and the same amount of exterior electrons. The alkali metals constitute one group in the periodic table. Some of the responses from this audience are captured in this video.
Read Also:
- How Many Houses Are In The World
- How An IVY League Turned Against A Student
- Compare and Contrast a Casual Friendship With a Close Friendship.
Periodic Table of Elements
The genius of the table lies in the fact that it allows a chemist to infer properties of one element from those of another in the same period or group. There are 118 different chemical elements, and they are all neatly laid out in a table called the periodic table.
In a periodic table, elements are typically listed from left to right and top to bottom in increasing atomic number (the number of protons in the nucleus), which also corresponds to increasing atomic mass.
According to Los Alamos National Laboratory, each horizontal row on the periodic table is called a “period.” And the number in that row shows the total number of orbitals for the elements in that row.
The nucleus of an atom contains protons and neutrons, and the electrons are arranged in mathematical orbitals around the nucleus.
An electron’s location and wavelike activity are described by these orbitals. Elements in period 1 have one available orbital for electrons to spin in, while those in period 2 have two, those in period 3 have three, and so on up to period 7.
Atomic elements with the same number of valence electrons (those in the outermost orbital shell) are grouped together into the same column on the periodic table.
Period Table is Not a Simple Grid of Elements Arranged Numerically
The elements of the periodic table are not simply laid out in numerical order on a grid. Elements are organised in the periodic table into horizontal rows, or periods, and vertical columns, or groups, with the periods being numbered in blue.
There are two systems of numbering that provide numbers for these categories, and they are not entirely consistent with one another. The International Union of Pure and Applied Chemistry (IUPAC) prefers the simplest presentation, in which the groups are only numbered 1–18.
However, in many parts of the world, it is customary to number the first two sets of ten (1A and 2A) and the last six sets of ten (3A-8A) and the middle set of ten (1B-8B) (but not in that order!).
While the IUPAC terminology seems to make things easier to remember, we will be using the current USA nomenclature throughout this article (1A-8A).
When “valence” and electron configuration are discussed in greater depth in Chapter 3, you’ll see why this was the best option. The arrangement of the elements in the periodic table is determined by their shared chemical characteristics.
Elements in the same Group (or column) of the periodic table tend to have similar chemical characteristics. Understanding the rationale for the periodic table’s current layout will become easier as we go over the elements’ individual qualities and how they mix with others.
Read Also:
- The Spanish Love Deception Barnes And Noble
- All Things Possible: Setbacks And Success In Politics And Life
- How To Tell The Difference Between Cellulose And Asbestos Insulation
Conclusion
The atomic numbers of the elements in the Periodic Table increase from left to right. Moving over a Period in the Periodic Table from left to right adds one proton to the nucleus (increasing the atomic number by one).
Groups 1 through 18 are vertical columns of elements with comparable chemical and physical properties. There are metals (blue) on the left, nonmetals (pink), and metalloids (yellow) along the zigzag line that separates the two groups. On the far right, you’ll see the noble gases.





